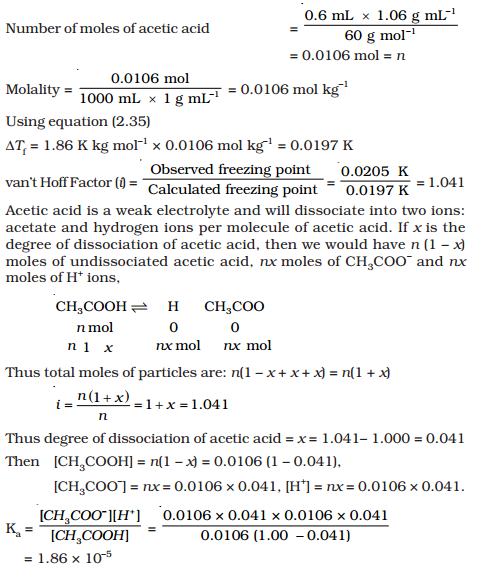
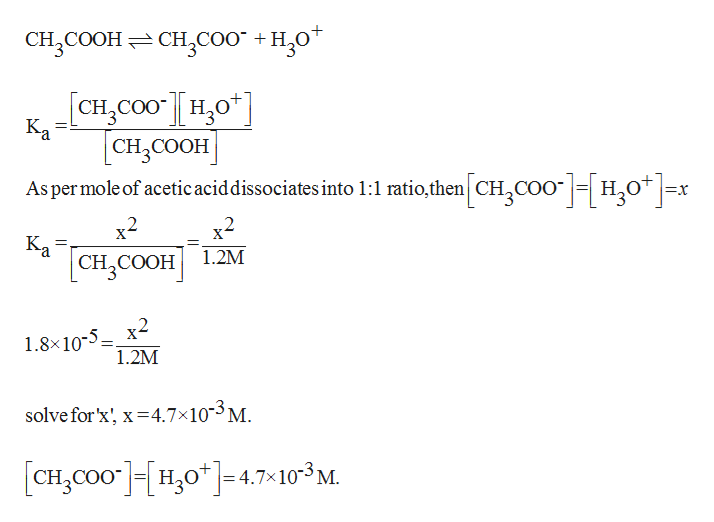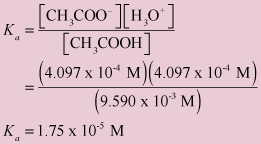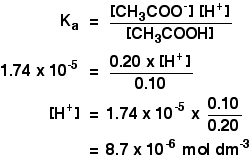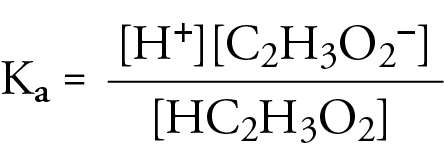A student prepared 0.10M acetic acid solution and experimentally measured its pH to be 2.88. Calculate Ka - Brainly.in
How to calculate the pH of 0.010 molarity acetic acid solution, if its dissociation constant is 1.8*10^-5 - Quora

Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question
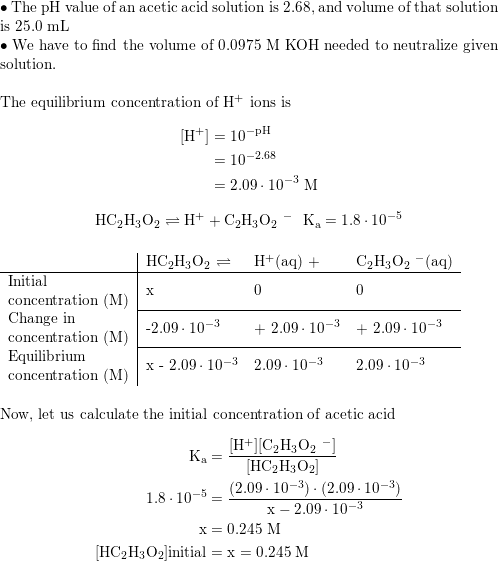
✓ Solved: Ka for acetic acid is 1.7× 10^-5 at 25°C. A buffer solution is made by mixing 52.1 mL of 0.122...
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://i.ytimg.com/vi/AufT6_CoFWY/maxresdefault.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
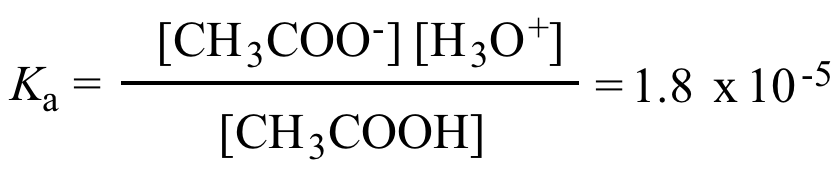
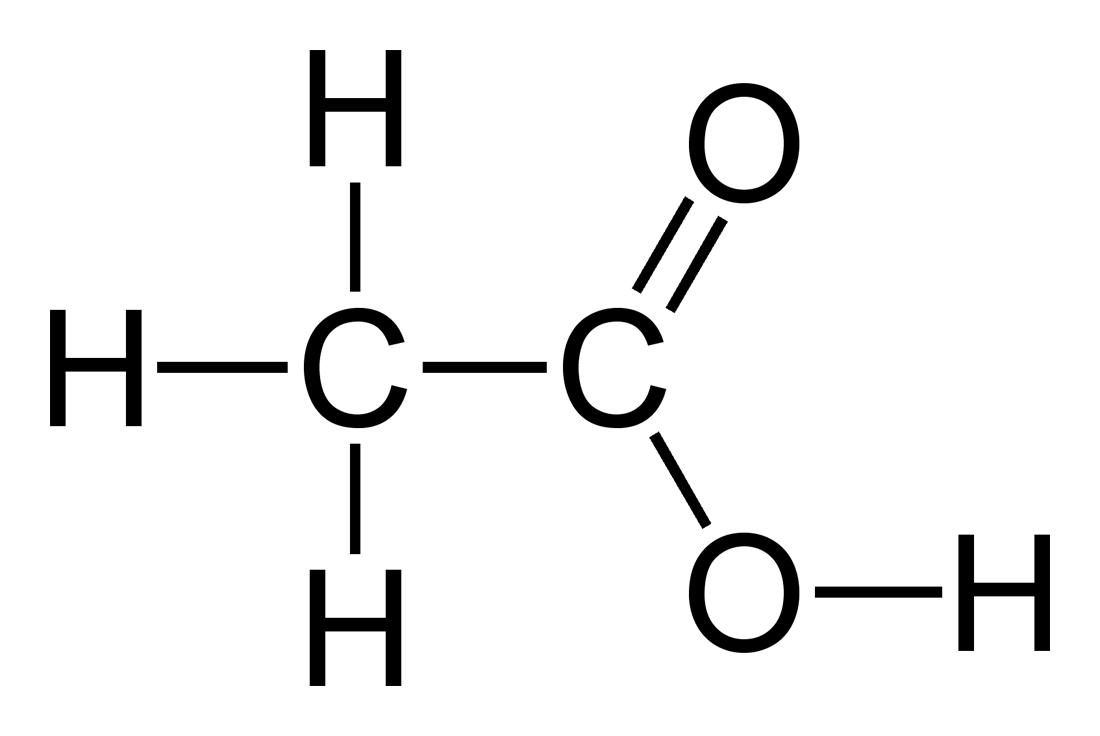
Illustrated Glossary of Organic Chemistry - Acid ionization constant (acid dissociation constant; Ka)

A 0 05 n sol of acetic acid is found to be 1 9+ ionised at 25 c calculate - Chemistry - Equilibrium - 13276973 | Meritnation.com