Supporting Information for “Relevant electronic interactions related to the coordination chemistry of tetracyanometallates. An
![Molecules | Free Full-Text | Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes Molecules | Free Full-Text | Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes](https://www.mdpi.com/molecules/molecules-28-04529/article_deploy/html/images/molecules-28-04529-g001.png)
Molecules | Free Full-Text | Synthesis, Structures, and Magnetism of Four One-Dimensional Complexes Using [Ni(CN)4]2− and Macrocyclic Metal Complexes
![Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/content_ck_images/ck_5a26e80693bfb.png)
Magnetic moment of [Ni(CN)4]2- is zero and that of [NiCl4]2- corresponds to 2 unpaired electrons Predict the geometry of - Chemistry - Coordination Compounds - 1176350 | Meritnation.com
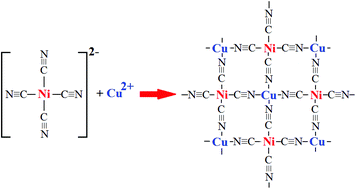
Chemistry and structure by design: ordered CuNi(CN)4 sheets with copper(ii) in a square-planar environment - Dalton Transactions (RSC Publishing)
✓ Solved: The complex ion NiCl4^2- has two unpaired electrons, whereas Ni(CN )4^2- is diamagnetic. Propose...
![Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species. - Zigya](https://www.zigya.com/application/uploads/images/chen12070397-a_5714962c868f8.png?t=1460966959418)
![The $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ ion, whic | Quizlet The $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ ion, whic | Quizlet](https://slader-solution-uploads.s3.amazonaws.com/3c5ed73f-36c2-4238-a711-7a222089df1a-1644081443307676.png)
![What is the hybridization for [NiCN4]2 ? What is the hybridization for [NiCN4]2 ?](https://byjus-answer-creation.s3.amazonaws.com/uploads/2.14.jpg_img_upload_solution_2022-05-30%2005:07:29.453226.png)
![Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com Write the hybridization and shape of the following complexe : [Ni(CN)4]2– - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:ffa0132b7d904ae4be8cc8b622fc0250.png)
![Ni(CN)(4)]^(2-) is diamagnetic , while [NiCl(4)]^(2-) is paramagentic Ni(CN)(4)]^(2-) is diamagnetic , while [NiCl(4)]^(2-) is paramagentic](https://d10lpgp6xz60nq.cloudfront.net/physics_images/FM_CHE_XII_V01_C05_E02_039_S01.png)
![Explain on the basis of valence bond theory that [Ni(CN)4]2&ndash Explain on the basis of valence bond theory that [Ni(CN)4]2&ndash](https://www.zigya.com/application/zrc/images/qvar/CHEN12070035-1.png)
![Solved Tetracyanonickelate(II), INi(CN)4]2 is a square | Chegg.com Solved Tetracyanonickelate(II), INi(CN)4]2 is a square | Chegg.com](https://media.cheggcdn.com/media%2F54e%2F54ef6548-6c25-4904-badf-967cfab1219a%2Fimage)
![coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange coordination compounds - Electronic configuration in [Ni(CN)4]2- - Chemistry Stack Exchange](https://i.stack.imgur.com/znHnV.png)
![Calculate the difference in magnetic moment of complexes [ Ni CN 4]2 and [ Ni Cl 4]2 . Calculate the difference in magnetic moment of complexes [ Ni CN 4]2 and [ Ni Cl 4]2 .](https://search-static.byjusweb.com/question-images/byjus/infinitestudent-images/ckeditor_assets/pictures/662819/original_B.jpg)

![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-2.png)
![Solved Tetracyanonickelate(II), INi(CN)4] is a square planar | Chegg.com Solved Tetracyanonickelate(II), INi(CN)4] is a square planar | Chegg.com](https://media.cheggcdn.com/media%2Ffd5%2Ffd59918a-32d3-4e6a-bac8-079dea008fcb%2Fimage)

![The geometry of [Ni(CN)4]2− and [NiCl4]2− ions are : The geometry of [Ni(CN)4]2− and [NiCl4]2− ions are :](https://search-static.byjusweb.com/question-images/toppr_ext/questions/345819_212776_ans_5a2a220d536a4adebbfad43e7ddf74b5.png)